Potassium Properties and uses of Potassium The boiling point for potassium is 1425°F Potassium has the melting point of 146°F A slivery color is. - ppt download

Potassium Vector Illustration. Chemical Element Substance Characteristics Uses. Boiling Or Melting Temperature Diagram. Alkali Metals Part And Ionic Salts Ingredient. Educational Labeled Infographic Royalty Free SVG, Cliparts, Vectors, And Stock ...

Difference Between Sodium and Potassium | Definition, Chemical Properties, Compounds, Isotopes, Similarities and Differences

Calculation the boiling point of a 1M aqueous solution (density 1.04 g mL^-1 )of potassium chloride (Kb for water = 0.52 K kg mol^-1 , Atomic masses: K = 39u, Cl =
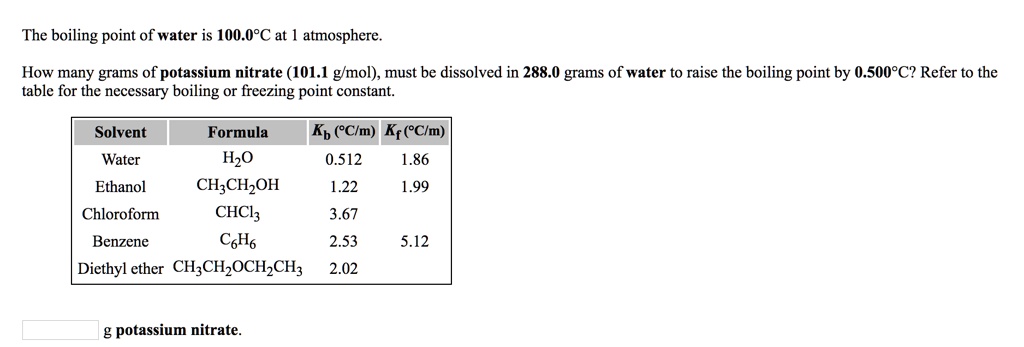
SOLVED: The boiling point of water is 100.0°C at atmosphere. How many grams of potassium nitrate (101.1 g/mol), must be dissolved in 288.0 grams of water to raise the boiling point by

Is the boiling point of 0.01 m potassium fluoride solution higher or lower than that of 0.01 m glucose solution? Explain. | Homework.Study.com
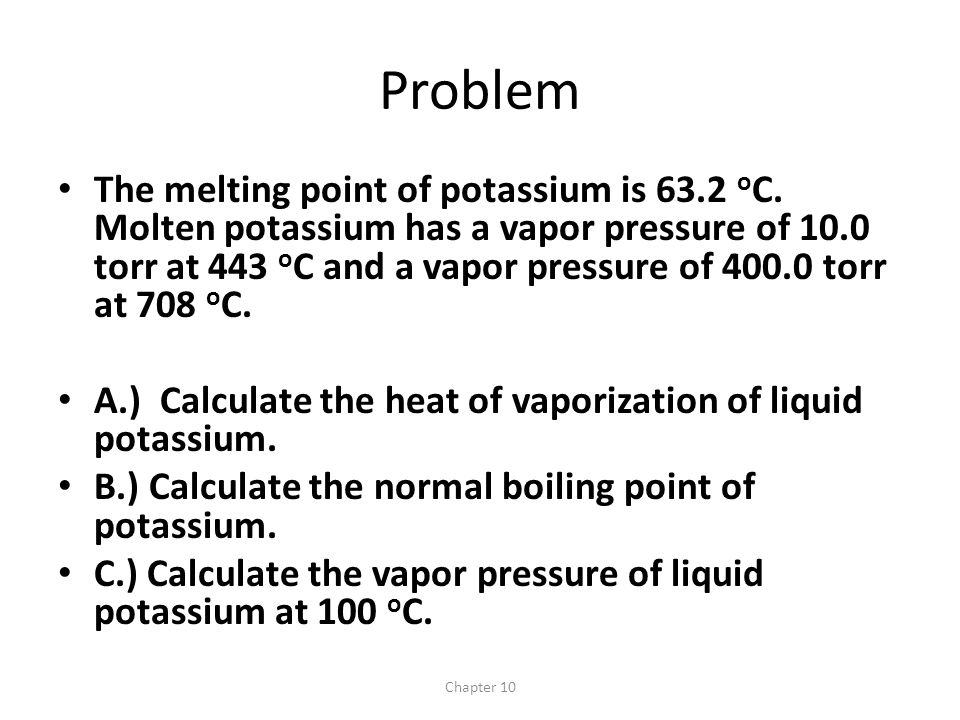
Chapter 10 Problem The melting point of potassium is 63.2 o C. Molten potassium has a vapor pressure of 10.0 torr at 443 o C and a vapor pressure of ppt download

Potassium Properties and uses of Potassium The boiling point for potassium is 1425°F Potassium has the melting point of 146°F A slivery color is. - ppt download

Calculate the boiling point of a 1M aqueous solution (density 1.04 g `Ml^(-1)`) of Potassium - YouTube

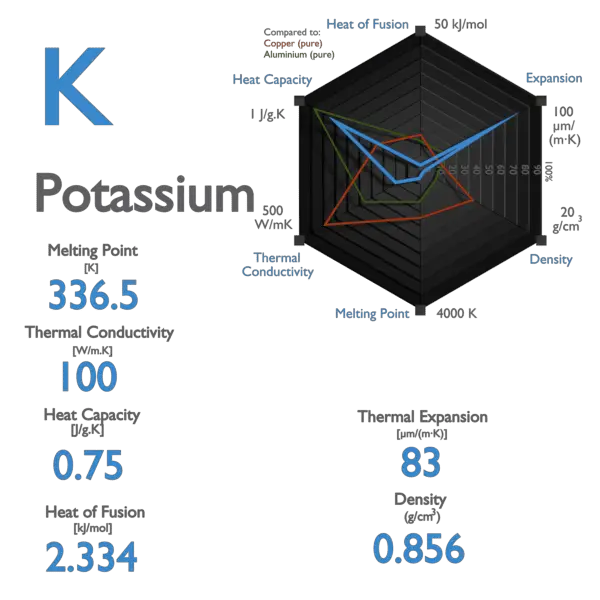
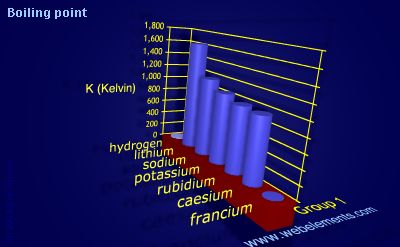
Chemistry Romania - >>Potassium< Group 1 Melting point 63.5°C, 146.3°F, 336.7 K Period 4 Boiling point 759°C, 1398°F, 1032 K Block s Density (g cm−3) 0.89 Atomic number 19 Relative atomic mass