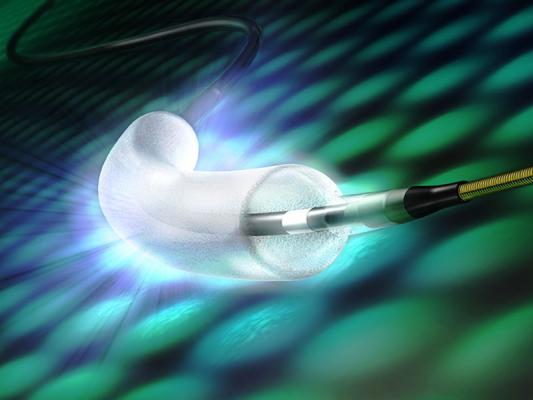
Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group - ScienceDirect

Advanced NanoTherapies' SirPlux Duo Drug-Coated Balloon Receives FDA Breakthrough Designation for Small Vessel Coronary Artery Disease

Concept Medical Granted FDA Breakthrough Device Designation for MagicTouch PTA Sirolimus Coated Balloon | DAIC
MedAlliance's SELUTION SLR drug-eluting balloon (DEB) receives FDA investigational device exemption (IDE) approval, making it the first limus DEB to be available to US patients
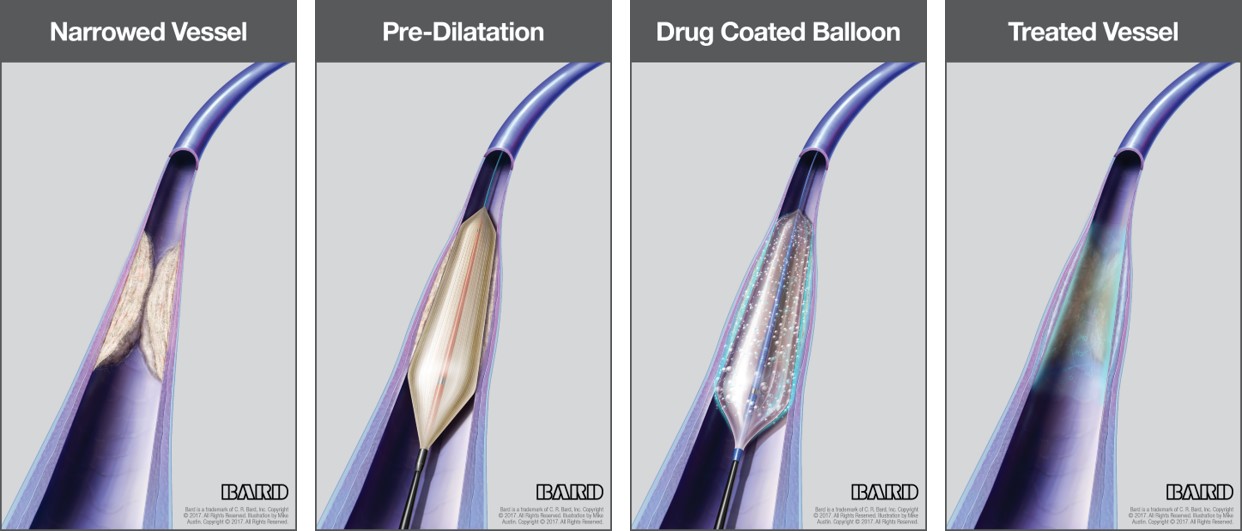
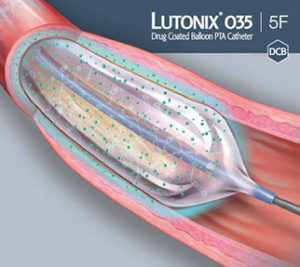
C. R. Bard Receives FDA Premarket Approval For The LUTONIX 035 Drug Coated Balloon - Medical Design and Outsourcing

Benefit and risk from paclitaxel-coated balloon angioplasty for the treatment of femoropopliteal artery disease: A systematic review and meta-analysis of randomised controlled trials - eClinicalMedicine

Chocolate Touch drug-coated angioplasty balloon for treatment of PAD receives FDA approval - Vascular News

FDA Approves Bard's Lutonix 035 DCB For Dysfunctional AV Fistulae Treatment | Medical Product Outsourcing

FDA panel votes against BD Lutonix drug-coated balloon for below-the-knee - Medical Tubing and Extrusion

FDA deems Orchestra BioMed's sirolimus-eluting balloon a breakthrough device for coronary restenosis | Fierce Biotech



_1653668541.jpg)