Mechanism for the carbaryl hydrolysis and 1-naphthol radical scavenging... | Download Scientific Diagram
Relative Stability of Formamidine and Carbamate Groups in the Bifunctional Pesticide Formetanate Hydrochloride

Hydrolysis susceptibility and carbamate formation for a low moisture-absorbing, siloxane-modified cyanate ester resin matrix (TC410) material used for composite space applications - Rafael J Zaldivar, Geena L Ferrelli, Hyun I Kim, 2022

Mechanism of Base-Catalyzed Amide Hydrolysis | Organic chemistry books, Teaching chemistry, Chemistry lessons
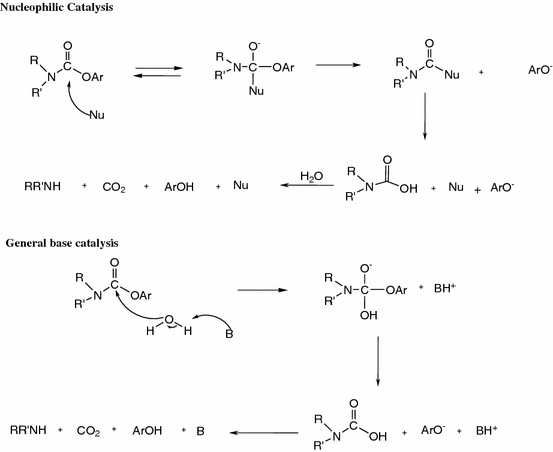
Alkaline hydrolysis of tertiary N-(2-pyridyl)carbamates. Contradictory evidence between nucleophilic and general base catalysis | SpringerLink

IJMS | Free Full-Text | Hydrolysis Mechanism of Carbamate Methomyl by a Novel Esterase PestE: A QM/MM Approach
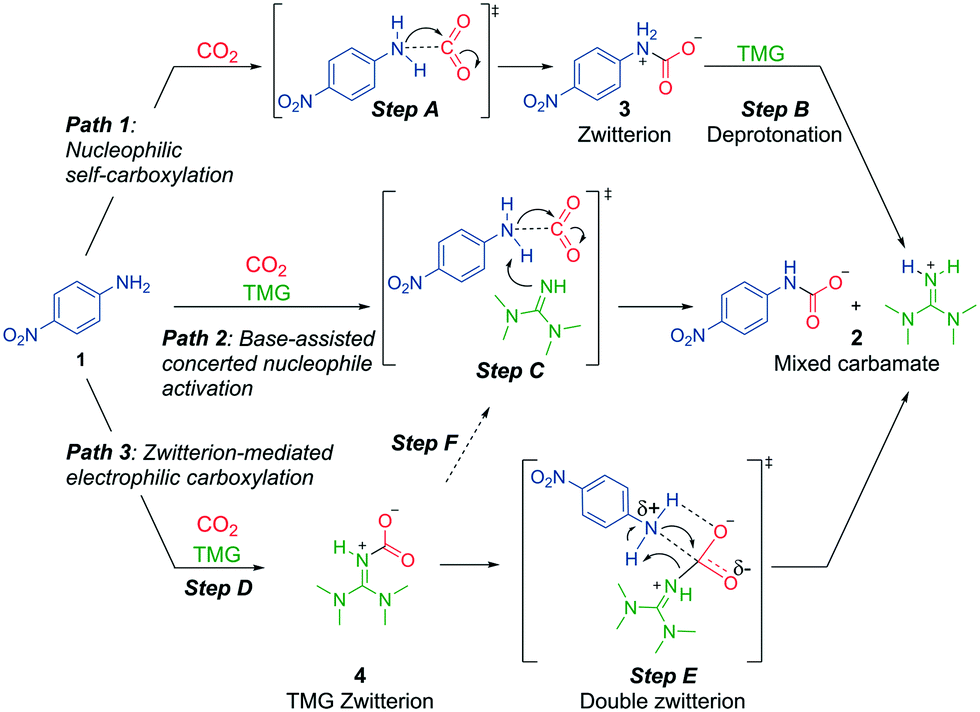
Mechanistic insights into carbamate formation from CO 2 and amines: the role of guanidine–CO 2 adducts - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/D1CY01433A
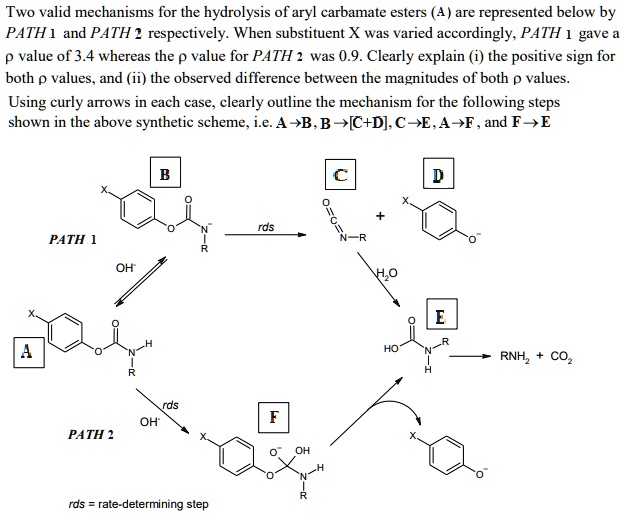
SOLVED: Two valid mechanisms for the hydrolysis of aryl carbamate esters are represented below by PATH and PATH 2 respectively. When substituent X was varied accordingly. PATH gave p value of 3.4

SOLVED: Question 3 (25 points): The carbamate containing compound (3) , releases the free amine rapidly under basic conditions: In contrast; a carbamate is extremely stable to base hydrolysis Describe an arrow
Carbamate group as structural motif in drugs: a review of carbamate derivatives used as therapeutic agents
Highly effective and specific way for the trace analysis of carbaryl insecticides based on Au 42 Rh 58 alloy nanocrystals - Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/C7TA01197K

SciELO - Brasil - Kinetics and mechanism of hydrolysis of benzimidazolylcarbamates Kinetics and mechanism of hydrolysis of benzimidazolylcarbamates

Figure 2 from Mechanism of action of organophosphorus and carbamate insecticides. | Semantic Scholar

Hydrolysis mechanism of esterases and amidases toward carbamate pesticides | Download Scientific Diagram

Mechanism of hydantoinase-catalyzed reaction. a Resonance forms for... | Download Scientific Diagram

Hydrolysis is the most commonly encountered drug degradation mechanism, both in solution and in the solid state. Use the structure of ethyl ethanoate below to illustrate the mechanism of acid-catalyzed hydrolysis of

Antibody Catalysis of BAc2 Aryl Carbamate Ester Hydrolysis: A Highly Disfavored Chemical Process | Journal of the American Chemical Society
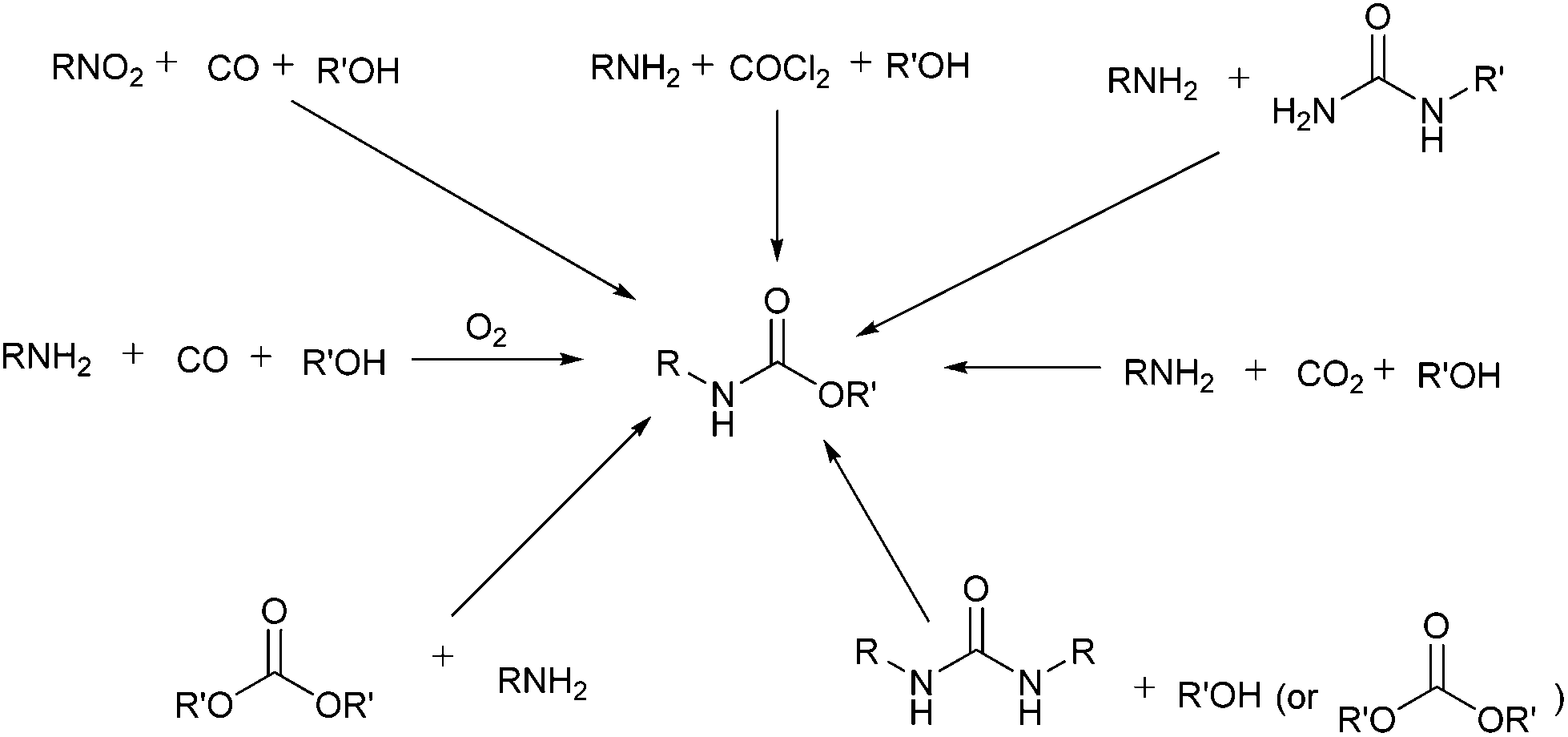
N -Substituted carbamate synthesis using urea as carbonyl source over TiO 2 –Cr 2 O 3 /SiO 2 catalyst - Green Chemistry (RSC Publishing) DOI:10.1039/C5GC01007A